The Discovery of Graphene
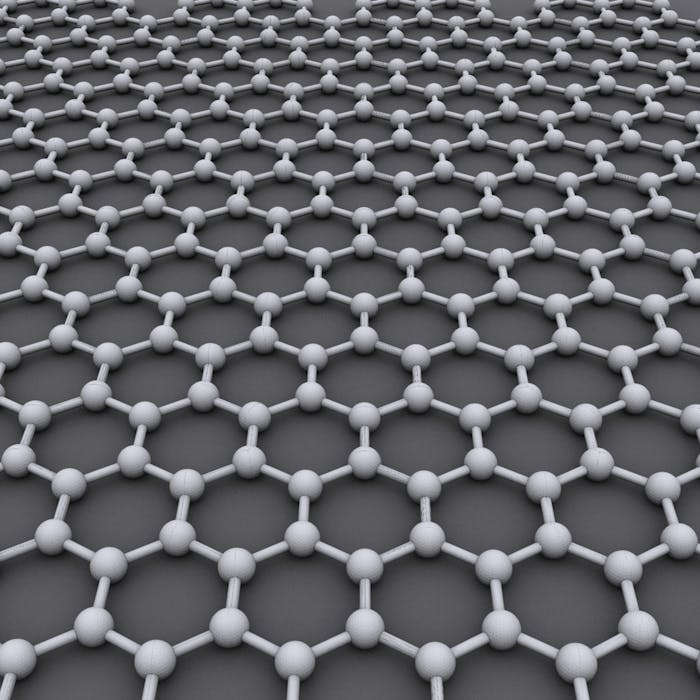
The "wonder" material graphene was discovered in 2004 at Manchester University. It is made of a single layer of carbon atoms arranged in a hexagonal lattice. Being a million times thinner than a human hair, it is the thinnest object ever created.
Scientists knew one atom thick, two-dimensional crystal graphene existed, but until 2004 no-one had worked out how to extract it from graphite.
Two researchers at the University of Manchester, Professor Andre Geim and Professor Kostya Novoselov, frequently held 'Friday night experiments' - sessions where they would try out experimental science that wasn’t necessarily linked to their day jobs.
One Friday, the two scientists removed some flakes from a lump of graphite with sticky tape. They noticed some flakes were thinner than others. By separating the graphite fragments repeatedly, they managed to create flakes that were just one atom thick. Their experiment had led to graphene being isolated for the very first time. The two scientists received the Nobel Prize in Physics for their discovery in 2010.
Scientists and inventors are excited by this new material due to its unusual properties. Not only is graphene lightweight and flexible, it is also the world's strongest material, being at least 100 times stronger than steel. It is an impressive conductor of electricity and heat, with applications in electronics and medicine. Other uses include heat dissipation, pollution detection, lightweight aircraft, cool shoes (literally), and much more efficient solar panels.
One of the most interesting potential uses of graphene is in graphene inks, which could make any surface (such as paper or clothes) "smart", touch sensitive and able to light up. Paired with a phone or TV over Bluetooth, it means you could answer your phone by tapping your clothes!
Further reading
Links to external websites are not maintained by Bite Sized Britain. They are provided to give users access to additional information. Bite Sized Britain is not responsible for the content of these external websites.
